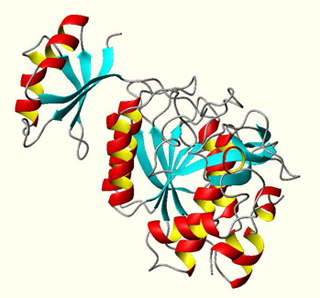
The enzyme Subtilisin BPN' has been the subject of numerous protein engineering studies. This monomeric protein has a substantial kinetic barrier to folding and unfolding, and is of great industrial importance. (Image courtesy of Dr. Philip Bryan, University of Maryland Biotechnology Institute. Used with permission.)
Instructor(s)
Dr. Melissa Kosinski-Collins
MIT Course Number
7.343
As Taught In
Fall 2004
Level
Undergraduate
Course Description
Course Description
This course is one of many Advanced Undergraduate Seminars offered by the Biology Department at MIT. These seminars are tailored for students with an interest in using primary research literature to discuss and learn about current biological research in a highly interactive setting. The instructor for this course, Dr. Kosinski-Collins, is a member of the HHMI Education Group.
Maintenance of the complex three-dimensional structure adopted by a protein in the cell is vital for function. Oftentimes, as a consequence of environmental stress, genetic mutation, and/or infection, the folded structure of a protein gets altered and multiple proteins stick and fall out of solution in a process known as aggregation. In many protein aggregation diseases, incorrectly folded proteins self-associate, forming fiber-like aggregates that cause brain cell death and dementia. In this course, the molecular and biochemical basis of the prion diseases, which include bovine spongiform encephalopathy (mad cow disease), Creutzfedt-Jakob disease and kuru will be examined. Also discussed are other classes of misfolding diseases such as Alzheimer's disease and Huntington's disease. The proteins involved in all of these disorders and how the proteins' three dimensional structures change during the course of these afflictions is covered as well as why prions from certain species cannot infect animals from other species based on protein sequence and structure. The course will then address possible detection methods and therapies that are under development to treat some of the protein aggregation diseases.


