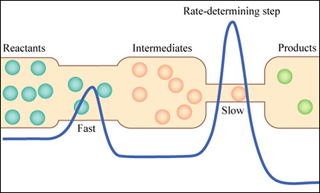
The reaction profile shows the potential energy at each step of a chemical reaction. (Image by MIT OpenCourseWare.)
Instructor(s)
Prof. Catherine Drennan
Dr. Elizabeth Vogel Taylor
MIT Course Number
5.111
As Taught In
Fall 2008
Level
Undergraduate
Translated Versions
Course Description
Course Features
Course Description
This course provides an introduction to the chemistry of biological, inorganic, and organic molecules. The emphasis is on basic principles of atomic and molecular electronic structure, thermodynamics, acid-base and redox equilibria, chemical kinetics, and catalysis.
In an effort to illuminate connections between chemistry and biology, a list of the biology-, medicine-, and MIT research-related examples used in 5.111 is provided in Biology-Related Examples.
Acknowledgements
Development and implementation of the biology-related materials in this course were funded through an HHMI Professors grant to Prof. Catherine L. Drennan.


