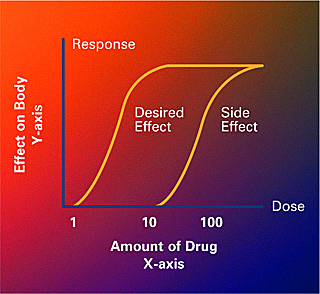
An example of a dose-response curve which shows how much of a drug causes a particular effect or side effect in the body (Crabtree + Company, National Institute of General Medical Sciences).
Instructor(s)
Prof. Charles Cooney
Stan Finkelstein, MD
Dr. G. K. Raju
Prof. Anthony Sinskey
MIT Course Number
15.136J / 7.547J / 10.547J / ESD.691J / HST.920J
As Taught In
Fall 2013
Level
Graduate
Course Description
Course Features
Course Description
This course serves as a description and critical assessment of the major issues and stages of developing a pharmaceutical or biopharmaceutical. Topics covered include drug discovery, preclinical development, clinical investigation, manufacturing and regulatory issues considered for small and large molecules, and economic and financial considerations of the drug development process. A multidisciplinary perspective is provided by the faculty, who represent clinical, life, and management sciences. Various industry guests also participate.
Other Versions
Other OCW Versions
Archived versions: ![]()


