Flash and JavaScript are required for this feature.
Download the video from iTunes U or the Internet Archive.
Description: An overview of the course and an introduction to the topic is given in this session.
Instructor: Lorna Gibson

Lecture 1: Introduction and...
The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high quality educational resources for free. To make a donation or view additional materials from hundreds of MIT courses, visit MIT OpenCourseWare at ocw.mit.edu.
LORNA GIBSON: OK, so what we're going to talk about in this course are materials that have a cellular structure. So they're all very porous. And typically they have low volume fractions of solids, like less than 30% solid. And we're going to talk about different types of cellular solids.
So one type are honeycombs. And this would be kind of your standard hexagonal honeycomb, something like that. We're going to talk about foams. And I'm sure you've all seen polymer foams. Here's an aluminum foam. We'll talk about other sorts of foams as well.
We're going to talk about some medical materials. And I brought in some bone samples, some trabecular bone samples here. I brought in a little tissue engineering sample here. And then, we're going to talk about materials in nature that have a cellular structure too.
So we're going to talk a little bit about wood. And I brought in a piece of balsa wood. I'll pass these around in a minute so you can play with them. This is the lightest wood. And I brought in lignum vitae. This is the densest wood. Lignum vitae is so dense that it sinks in water.
And I have a couple of projects right now on natural materials. So I thought I'd talk a little bit about those too. We have one on bamboo and making structural products out of bamboo. So this is like a beam made out of bamboo. And this is a piece of oriented strand board made out of bamboo. So the same way you have like wood oriented strand board, you can do the same thing with bamboo.
And we have a project that involves this, so I thought talk a little bit about that later on in the course. So we'll talk a little bit about the processing, how you make these materials, the structure. We'll talk a little bit about how we do the modeling.
So we're going to start with the honeycombs, partly because they have a nice, simple unit cell that you can repeat and you can analyze fairly exactly. And then we're going to talk about modeling the foams.
And then once we've got the modeling background, so we're going to get equations describing the mechanical properties, the stiffness and the strength, once we've got that, then we can apply it to lots of things. So we can apply it to understanding the trabecular bone, the tissue engineering scaffolds, or how cells interact with scaffolds.
We're going to look at energy absorption in foams because they're good for absorbing energy. We're going to look at a lightweight sandwich panels as well.
So we're going to look at all these different things. And I have some slides I'm going to go over today that have more pictures of all of this. And I'll pass this around to a sec, but let me go through the logistics first.
So this first hand out has a few of the kind of details. There's two books that I've coauthored with colleagues. The one on cellular solids, if you wanted to buy a book, is probably the most relevant one to buy. If you don't want to buy it, you certainly don't have to. I'm sure the library has multiple copies of it.
And the other more recent one is called Cellular Materials in Nature and Medicine. And that's a little more specialized. And again the library has that. So you don't need to run out and buy that. You can just get it there, but let me just mention there's that reference and it might be helpful.
OK, so let me talk a little bit about the projects. What we do in this class is everybody does a project. And I like for you to do it in pairs, just so that you have somebody to work with. I think it's nice to have somebody to do it with. And the project has to be something on cellular materials, but I really leave it pretty open ended what the project is. It's really up to you to decide what you'd like to do.
And to give you some idea what people have done, people have done all kinds of stuff in the past. So people have worked on negative Poisson's ratio honeycombs. I didn't bring any of those in with me, but you can design these honeycombs so that they have this property that when you push it this way-- instead of if you make the strain smaller in this direction, it gets wider in this direction normally, but with negative Poisson's ratio materials, it will contract in that direction. So I've had people do projects on that.
I've had people work on osteoporosis. I had a group once who worked on elephant skulls and I brought part of their project with me. So it turns out elephant skulls have a sort of porous layer to them. And this is-- they have these large pores in the top of the skull. I don't have a whole skull, but this is part of it.
And what they did was they had heard that elephant skulls have these pores and that the pores somehow affect sound transmission and how the elephant hears. And so they wanted to do a project on that. And that was really all they knew to start with, elephants have pores and we want to do a project on it because it's cool.
So I helped them put together this project. We went up to Harvard. We went up to the Museum of Comparative Zoology where they have elephant skulls, which are like about this big around, huge skulls. And some of them had the outer part of the bone was broken and you could see these big pores. So they got some nice pictures of elephant skulls.
And then they found that the University of Texas at Austin has computer tomography images of all sorts of bones of different animals. And sure enough, they had elephant skulls. So they got the file for the micro CT image, which gives you the sort of 3-D picture of the skull. And then they use that to 3-D print small versions of the skull.
So that's what this is, they printed smaller versions. And these were just a couple of slices that I got from them at the end project. And then what they did with one of the skulls, they suspended it from a wire, and they took speakers, and they had sound that vibrated the skull, and then they put an accelerometer on the skull and they measured the vibration response of the skull.
And they also made, I think, it was a dolphin skull, which did not have these pores, and they kind of compared the vibration response from the sound for these two skulls with two different structures. So that was a project for this class.
People have worked on tissue engineering scaffolds. Often there's people who work on food foams. So one year, people worked on bread. They did bread processing. They made bread by having different amounts of yeast, different rise times, different ingredients. And they made bread.
And I have a little-- I like these historical things. And I have a little thing here I wanted to show you. So the people in 3032 last year, last fall, will know that I went to England in November. And I went to the Royal Society for an editorial board meeting. But I also went and looked at their archives.
And one of the things they showed me was this article, which is in the sort of an original, sort of archives of the Royal Society from 1600s. And it's by a guy called John Evelyn. He's famous for writing a book called Silva about trees and wood. But he's written this article here. And I love the title. It's "The Several Manors of Making Bread in France, Where by General Consent, the Best Bread in the World is Eaten," by Mr. Evelyn.
So here we have in the Official Royal Society bread making science. And in fact, the article was several pages long. There was quite a lot on bread and how you make it in France, where the best bread is eaten.
So if you want to do something on food foams, people have done meringue before, various things. They look at typically changing something about the recipe, or the composition, or the processing, or the baking. And they look at the structure. Sometimes they look at mechanical properties too.
So anyway, there's a whole list of things there. You can think of other things. If you look through the books, you might get some ideas as well for projects.
So what I'd thought I'd do next is I wanted to just kind of give an overview of what we're going to talk about. And I've got a bunch of slides. Let's see I forgot some things, because I do that. Books, yeah, OK, let me pass some of these things around so you get to play with them too.
So here's some honeycombs. I don't know if we can pass all these things around because it's a little unwieldy. So this is an aluminum honeycomb. I like bringing toys in. These are little rubber honeycombs. This is a little ceramic honeycomb. This is a little paper honeycomb.
And let's see, we have some foams here. So here's a metal foam and a ceramic foam. And this is a sort of, not quite a foam, it's made of hollow spheres by centering hollow spheres together. So you can play with that.
And what else do we have? We have little lattice things here. So here's a sort of 3-D lattice material. So this has sort of a cellular structure, but it's very, very regular. It's not like a foam. So that's called a 3-D lattice material or sometimes a 3-D truss.
And what else should we pass around? We need to do the bones. Here's the wood. You can feel how different the densities of the two woods are. I would like these all at the end because I show these around for different classes. Here's some bone, you can see of the bone looks like the foam. And this is a tissue engineering scaffold for generating skin.
All right, so while those are getting passed around, I'll talk a little bit about what we're going to cover in the class with some slides. OK, let's see, should I dim the lights? Would that be a good thing, Craig, if I dimmed the lights?
CRAIG: They're kind of preset, you can try maybe two.
LORNA GIBSON: OK. Doesn't seem to-- there we go. How about that? That OK? All right, so I like these historical things. So this is a picture of Robert Hooke's drawing of cork from his book Micrographia. And he was the first person who used the word cell to describe a biological cell. And it comes from the Latin cella, which means a small compartment.
So you can think of the cells as small compartments. That kind of makes sense. And he kind of very modestly says, "I no sooner to discerned these, which were indeed the first microscopical pours I have ever saw, but me thought I had with the discovery of them perfectly hinted to me the true and intelligible reason for all the phenomena of cork."
So he's saying by looking at the structure of cork, he thinks he understands everything about the properties of cork-- very modest. But in fact, this is kind of the foundation of material science. So material science is all about looking at the structure of materials and trying to say something about the properties of the behavior of the materials. And that sentence kind of sums that up. So that's why I like that sentence.
So what we're going to do is look at different kinds of cellular materials. We'll look at engineering ones. And these we typically refer to as honeycombs or foams. Honeycombs have two dimensional prismatic cells, while foams have three dimensional polyhedral cells.
And we'll look at applications for the honeycombs and foams in things like lightweight sandwich panels, in energy absorption devices, and things like thermal insulation. We'll talk a little bit about the thermal properties. We're also going to talk about cellular materials in medicine, so trabecular bone. We'll talk about osteoporosis and how loss of bone reduces the strength, and how you might estimate that.
We'll talk about tissue engineering scaffolds, something about their mechanical properties. You may think the mechanical properties aren't probably the most important thing. But in fact, the mechanical properties do have some effect on how the cells interact with the scaffold. So we'll talk a little bit about cell scaffold mechanics.
And then we're going to talk about cellular materials in nature at the end of the course. So we'll talk a little bit about honeycomb like materials, like wood and cork, and foam like materials, like the trabecular bone. There's also a type of tissue in plants called parenchyma that looks just like a foam. And there's some sponges that have some interesting features.
And often, in nature the cellular material appears in combination with some solid material. And so it's sort of a structural component. And you can see sandwich structures in nature, leaves and skulls. You can see materials that have density gradients, palm stems and bamboo are examples of that.
And you can see materials that have cylindrical shells with compliant cores, and things like plant stems and animal quills are like that. So that's kind of the range of materials that we're going to talk about.
And one of the interesting things about cellular materials is that you can make cellular materials out of almost anything now. And this is really a huge range of materials and lots of different applications for this.
So one of the fundamental things we're going to do is look at the mechanisms by which the materials deform and how they fail. And we'll use a structural analysis to obtain the bulk mechanical properties, so things like the stiffness, the moduli, the strength, the fracture, toughness.
We'll look at how you can control the design of the microstructure to get the properties that you might want, and also how you might select for the best material for a given engineering application in engineering design. So we're going to get those three things.
So let me start by showing you some more examples of engineering cellular solids, and in particular, some micrographs. So that you can see what it looks like on a small scale too.
So these are the honeycombs. These are the sorts of things that I'm passing around now. The aluminum one, paper resin, and the ceramic ones. The aluminum and the paper resin ones are typically used in the cores of sandwich panels. And the ceramic ones are used in the catalytic converter in your car.
So what they do is they block off every other cell at one end and then the opposite cells at the other end and exhaust gas from your car is forced to go through those channels in the honeycomb. And the walls, if you look at that triangular one, those triangular walls themselves are porous, and they're coated with the catalyst, which is platinum. And that forces the exhaust gas through the wall in contact with the platinum, and then comes out the other end. And they're ceramic, obviously, because the gas is hot and they need something that has a high thermal resistance.
So these are some examples of honeycombs. These are some examples of engineering foams. When I tell people I work on foams, they always think of polymer foams, like polystyrene or something. And there's lots of polymer foams. But you can actually foam any materials now.
There's metal foams. There's ceramic foams, and glass foams, carbon foams, all sorts of foams. So those are some examples. You can see when you look at these images here that the foams have a low volume fraction of solids, like if you look at say this polyethylene one here. Say we look at this guy up here, then you can see there's not much solid, there's a lot of gas. So the volume fraction of solids is fairly low on that foam there.
So one of the things we're going to talk about is how the volume fraction of solids affects the properties. You can also see on the top left and the top right, the top left one has what we call open cells. There's just edges along the polyhedra, there's no faces over the membranes. And the right hand one is a closed cell foam. So there's like membranes that cover the faces of the cells.
So we're going to talk a little bit about the differences and the behavior of open cell and closed cell foams too. These are food foams. So I've already said you might want to do a project on food foams. And these are just some examples of different kinds of foods that are in fact foams. And it turns out the food industry spends quite a lot of time and effort thinking about the mechanical properties of food. And it turns out if the texture of the food isn't right, then people don't like the way it feels in their mouth. There's something they actually called mouth feel.
So it turns out if your cereals too soggy, it's icky. If it's too crunchy, it's icky. So it's sort of a happy medium. And food companies spend quite a lot of time and money worrying about the mechanical properties of food.
This is an example of showing that the cells could be antisotropic, the cells could be elongated in one direction. For instance, in the top one on the polyurethane foam. And if they're elongated in one direction, it's not too surprising, you might have different properties in that direction from the plane perpendicular to it.
And then the bottom images is of pumice. Pumice is a volcanic rock. And you can see how the pores are kind of flattened out there. And they're flattened out because that was once molten lava. And the molten lava was flowing down a mountain side of a volcano. And as it flowed, it got sheared. And the shape of those pours reflects the shearing as the molten lava flow down the volcano.
And so this kind of sort of stretched out cell shape is going to give you antisotropic properties, different properties in different directions.
This is the 3-D truss that I'm passing around. I don't know if it's exactly the same one, but it's a similar one. And these trusses are triangulated structures. And we'll talk a little bit about their properties too. And then we also are going to talk about some applications.
So obviously, these materials are mostly air. And that gives them a low weight. And that means they're often used in structural sandwich panels as the core of the panel. And these panels have stiff faces separated by a lightweight core. And the idea is to make it a little bit like an I-beam.
So the way you have the flanges on the I-beam, the faces are like the flanges, and the porous core is like the web of the I-beam. They can also undergo large deformations at relatively low stress. And that means they can absorb a lot of energy. So if you think of the energy as the area under the stress strain curve, if there's big strains and big deformations, then there's going to be a large area.
And that sort of energy absorption occurs at a fairly low stress. So typically, when you want to absorb energy, it's not just how much energy you want to absorb. You have to do it without actually breaking the thing you're trying to protect. So you don't want to generate high stresses as you go along, and foams are good at this.
Foams are also good at being thermal insulators. They have a low thermal conductivity. And that's because they're largely made of gas and the gas has a lower conductivity than the solids. So that gives them a lower conductivity. And they have a large surface area. And the smaller the pore size, the bigger the surface area per unit volume. And that makes them good for things like carriers for catalysts. And that's why they're used for these catalytic converters too.
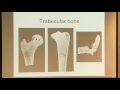
OK, so here's some examples of cellular materials in medicine. So here's some examples of trabecular bone. Trabecular bone exists at the ends of your long bones. So say in your hip or in your knee. It also exists in your vertebrae in the middle of the spine in the vertebrae there. And it also exists in your skull.
And you can see it's a porous type of bone. It looks very similar to the foams and the sorts of mechanical models we make for foams can be applied to the bone as well. And so that's one of the things we're going to do later in the course.
These are two slides showing what happens when people get osteoporosis. The left hand slide is from a 55-year-old female to the same bone, the same slice. And the right hand one is from an 86-year-old female. This thing here, row star over row S, that's the relative density, the density of the bone divided by the solid that it's made from. That's the same as the volume fraction of solids.
And so on the normal bone on the left it's about 17% solid. And on the osteoporotic bone on the right it's, about 7%. So you can kind of see the bone density has gotten lower, partly by thinning of the struts, but partly by resorption of the struts, as well. And obviously the one on the right is going to have a lot lower strength than the one on the left.
These are micro CT images of bone. And again, you can see how the structure looks different at different relative densities. The one on the left is sort of in the middle at around 11% dense. The one in the middle is the most dense 25%. And the one on the right is 6% dense. So it's not too surprising that the one on the right would have a much lower strength from the other ones. And we'll look at how we can model that.
This is just showing some deformation in bone. I have a colleague, Ralph Mueller who's got a micro CT machine, which allows you to do compression tests in the micro CT. So he can make these sort of images where he scans it at zero strain. He compresses it a little bit. He scans it again. He compresses it a bit more. He scans it again.
And he these are stills from his images, but he makes animations from this. And if you look at the top right up here, you see these struts here. They're pretty straight in this one. They're a little bent and starting to buckle here. And then if you look at that one strut there, you can see how it's buckled right over. So you can look at the deformation mechanisms by looking at the CT scans and things like that.
People are starting to think about using metal foams for coatings of orthopedic implants. So one of the issues with implants is that say you have a hip implant or a knee implant, you remove the bone that's preexisting, and then you replace it with some sort of implant. Typically, the implant has a stem that fits into the hollow part of the bone and then has a sort of joint piece to it that fits into the joint. And you can get some loosening of the stem in the remaining bone.
And one idea is that you use porous coatings to minimize that. And right now, typically what they do is center beads, metal beads on to the stem. And another idea is maybe you could use a metal foam. And these are some different types of metal foams that people are looking at.
Another type of cellular material in medicine is a tissue engineering scaffold. And this just shows some different examples made by different processes. And we'll talk more about these later on in the course. This one here at the top left is a collagen based one made by a freeze drying process. And I don't know if you saw MIT's website yesterday and today, Ioannis Yannas was the one who developed this. And he's just being inducted into the National Inventors Hall of Fame. And this is what he really was inducted for is he's invented a skin-- well, he calls it a dermal regeneration template for regenerating skin, mostly in people with serious burns.
Then these are some other sorts of scaffolds that are made by different processes. This is by a sort of rapid prototyping process here. The bottom two, these are kind of interesting. These are actually the extracellular matrix in the body. And they've had all the cells removed from it. So these tissue engineering scaffolds are really designed to mimic the extracellular scaffold in your body or extracellular matrix in your body.
And you can see how when you remove the cells, the structure of those two things looks a lot like a closed cell foam. So that's the kind of structure you're trying to replicate.
We'll look a bit at cell mechanics. This is a cell contraction of a scaffold. So here these sort of very thin transparent bits of the collagen based scaffold, and this is a fiber blast on it right here.
And I had a student, Toby [? Fryman, ?] who worked with me and Ioannis Yannas on this. And you can see from the video the cell is actually contract in the scaffolding making it deform. And you can calculate what forces the cell must be imposing on the scaffold by knowing something about the geometry of the struts and how the cells attached. And then it's going a little bit more. So we'll talk more about that.
And then there's a final picture down here, where you can see these two, the two points up here and down here have now been brought pretty much right together. So we'll talk about that in more detail.
We've also done studies on cell attachment and how that attachment rate or the amount of cells that attach is related to the surface area, the surface area per unit volume. So these are just some tests from that done by [? Fergal ?] O'Brien, a post-doc that worked with me.
We've also done some studies on cell migration. And Brendan Harley was the student who did this. And he stained the cells with one stain and he stained the scaffold with something else. So red are the scaffold and green are the cells.
And then he used a confocal microscope to track the cells. And he tracked the cells and where they moved versus time. And if he has the location at different times he can get the velocity.
And one of the things he did was he changed the stiffness of the scaffold and he found that the migration speed depended on how stiff the scaffold was. So he was looking at sort of interactions between mechanical properties of the scaffold and behaviors like cell migration.
And then we're going to look at materials in nature. So here is wood. So you can see the cellular structure of wood. It's a lot like the honeycombs. It has sort of a prismatic structure. That one happens to be cedar, but other woods look similar.
Now, this is balsa wood. And this is showing just how the balsa deforms. I think this was loaded from top to bottom. And this is at zero load. And then this is more load, and more load, and more load.
And if you look at that cell there with this little kind of tear in it here, that's the same as the cell down here, and that's the tear there. So you can see how the cell walls bend and how they deform. And you can model that using the honeycomb models.
This is just another image showing actual failure of wood, buckling of the cell walls. This is cork. So these are modern scanning electron micrograph of cork. And one of the interesting things is the cork cells have these little corrugations.
You see how they're not flat, they have little kind of wrinkles in them. And that gives rise to sort of an interesting property of cork. If you take cork and you load it. So here we were pulling it in the direction of these arrows, pulling it like along this direction here. And again, this is the same set of cells. That tear there is the same as that tear there.
And you see all these little corrugations here, they've all straightened out when we're pulling on it. And the Poisson's ratio of the cork is zero. It's kind of like a bellows. Like if you had an old camera, or you have an accordion bellows. If you pull the bellows in and out, it doesn't get any wider this way or the other way.
You're just sort of opening the bellows and closing the bellows. And the cork cells are doing kind of the same thing. And it gives them this Poisson's ratio of zero. Which it happens is one of the things that makes it easy to get the cork into your wine bottle, because as you're pushing on it, it's not pushing out in all directions. This is only for this one direction, but it's not pushing out in all directions.
These are parenchyma cells in plants, in carrots and potatoes. All those little blobs in the potato, those are starch blobs. This is called a Venus flower basket sponge, and Joanna Aizenberg, at Harvard, has studied this quite a lot. This has a hierarchical structure.
If you look at the overall sponge and then you look at each of the sort of struts that make up the lattice, that too has a hierarchical structure. And she's looked at the optical properties of this glass sponge. It's kind of a beautiful thing.
And then there's some cellular structures in nature as well. There's sandwich structures. There's density gradients. And there's tubes with a cellular core. So here's some examples of that.
Here's the iris leaf, you know the iris plant has these long kind of leaves that stand up like this. And it's just like a sandwich panel. The parenchyma are kind of like a foam in the middle here. And there's very dense fibers called sclerenchyma that run up and down the length of the leaf. And they're like fibers in a fiber composite.
And here's a bull rush or a cat tail leaf. And they're like little I-beams almost. It's like a whole series of little I-beams. And again that's sort mechanically efficient. These are examples of sandwich structures in bird skulls. Some of you know I'm a birder. So I sort of sneak in bird stuff from time to time.
But you can see how these birds skulls are all sandwich panels, and obviously birds want to minimize their weight for flight. And this is one of the ways that they do that. This is a horseshoe crab, sort of similar kind of thing. This is from Mark Myers in San Diego. He did a study on the crab in its shell as a sandwich.
And this is the ever so handsome cuttlefish. And the cuttlefish has something called a cuttlefish bone. This is the bone here and the bone is made up of these kind of sandwich type structures. The cuttlefish is related to octopus and squid and things like that.
And it's hard to see in this picture here, but these little things here are actually like little tentacles. There's several tentacles that it eats stuff with. The cuttlefish is actually a mollusk. All those things are mollusks. It's called a fish, but it's not really a fish.
And here's an example of a natural material that has a radial density gradient. Have you ever noticed if you look at a palm, like you see those pictures of Hollywood in LA, and the palm trees, you know, they line the street.
But they're all about the same diameter from the bottom to the top. And when you think of like an oak tree, it's not like that. It's big diameter at the bottom, skinny diameter at the top. So when wood grows, the wood has more or less the same density in the bottom and at the top. So as it's growing, the density is more or less the same.
And it resists the bigger loads from getting taller by adding circumference. So it gets wider and wider as it gets older and older. But palms don't do that. They come out of the ground a certain diameter. And most palms just grow that same diameter. As they get taller and taller, you can imagine there's wind forces, and different kinds of forces are on it, the stresses get bigger and bigger.
And the way they resist those is that the cell walls get thicker, and they preferentially get thicker on the outside. And if you think of moment of inertia, remember moment of inertia is increased more with the material on the outside of a beam. And that's kind of what the palm is doing.
So if you look at young cell walls and old cell walls, here's some SCM pictures of young ones and it's sort of skinny. And SCM pictures of older ones, and the cell wall has gotten thicker.
So we're going to talk a little bit about that. And it turns out, this is an incredibly efficient way to deal with getting taller and needing to resist bigger loads.
Another material that has a radial density gradient is bamboo. So this again shows these sort of dense sclerenchyma fibers. Do you see these kind of dense parts here? And you can see there's not very many of them here. And there's more and more as you get towards the outside there. So there's a density gradient there. So we'll talk about that.
And some plant materials have a cylindrical shell with a compliant core. Plant stems or commonly like. This is a milkweed stem. And you can see it's got these sort of fibers that are almost completely dense. And then a sort of lower density core, cellular core, here, and a void in the very middle.
And you can show that that core helps prevent local buckling. So if you take a drinking straw and you bend it, you get that kinking kind of failure. You can show that having this sort of foam like core in the middle helps to resist that.
Imagine you have a drinking straw and now you put foam in the middle. It's going to be harder to get it to kink like that. So that's what the plants are doing. Animal quills do the same thing. That's a porcupine and a hedgehog quill.
And all of this stuff is in these two books. So it doesn't really matter to me if you go out and buy the book. I don't make very many much money on these books. So this is not an income producing thing. But those are the books that all these pictures have been taken from.
All right, so I think that's my sort of introduction to the class. Are there any questions about how we're organized or what we're going to be doing? Are we good? It's OK? OK. So then I think what I'm going to do for the rest of the time is start the next section of the course which is on processing of cellular materials.
Now, I have another little hand out here. So I don't know if I'll remember to do this for every lecture, but I like to have a little outline, that partly makes me be organized. So it's just a little outline for the lecture.
AUDIENCE: [INAUDIBLE].
LORNA GIBSON: You asked me to do what?
AUDIENCE: Put the room light back up.
LORNA GIBSON: Put the what up?
AUDIENCE: Lights.
LORNA GIBSON: Oh, the lights. I'm going have another set of slides though. So let me get out of the intro slides. I know I have another set of slides. So I'm going to just leave the screen up. And kind of put stuff on the board and talk about the slides.
AUDIENCE: That's fine.
LORNA GIBSON: You're good? OK. OK. So I wanted to talk a little bit about processing of cellular solids and then, next time we'll start talking about the structures. It seemed good to talk about the processing before we got to the structure.
So I'm going to talk a little bit about honeycombs and how they make honeycombs, and then foams, and then lattice materials. Yeah?
AUDIENCE: The slide you're showing with the the shell with the foam inside it, are there techniques for analysis of it?
LORNA GIBSON: Well, I don't think we're going to get into all the gory details, but I can certainly give you references. That's something that one of my students did at one point. And in fact, I've been collaborating with Jennifer Lewis up at Harvard and she has a student who's making sort of cylindrical shells with foams out of ceramic foam. So he's 3-D printing sort of with coaxial nozzles a cylindrical shell that's pretty solid. And then a ceramic foam on the inside. And that's one of the things he's playing around with. So he's looking at ways you might make engineering versions of that.
So I wanted to start with looking at honeycombs and how they make honeycombs. And I thought what I'd do is I've got some slides. And I'm going to talk about the slides. And then I'll put some notes on the board to kind of describe what we're doing, OK?
So this is the first sort of slide on the honeycombs. And the two main techniques that they're made by, especially those aluminum honeycombs and paper honeycombs that I passed around, one technique is an expansion process.
So what they do is they take flat sheets of some metal, say aluminum, and they put little stripes of glue on it in different places. So these little kind of specialty things are where the glue goes. And then they stack those guys up in a sort of particular arrangement.
And then what they do is they pull it all apart, kind like a paper doll thing. They pull it all apart and when they pull it apart, they get the hexagonal shape. So let me just show on the board how you do the gluing and how that works.
So they would start with some sheets. Say we start with two sheets like that. They'd put some glue down, say there. And then there's a gap. And then they put glue on the opposite side over there. And then there's another gap. And then they put glue there.
And then they do the same positions but the opposite sides on the next sheet. So they do that. And then if you glue those together-- well, let me do another one. Maybe do a couple more. So then if I do one like that, it's glued there, and there, and there. That guy gets there, and there and there.
And when glue that-- when you push that together and then take it apart, you've got something that looks like this. So say I call that one, two, three, and four. Then this would be 2 and 3. OK, so this thing here is that. Where it's not glued, you get them doing that. And then it's glued again down here. And so you get this kind of pattern.
And one of the things about these honeycombs that are made by the expansion process is these inclined walls have a thickness t. And because there's two sheets up here, the vertical walls have a thickness 2t. So that's typically what you see in the commercial honeycombs that are made by this way.
And this process is used for aluminum honeycombs, for paper resin honeycombs, for Kevlar honeycombs. And I'll just say note that the inclined walls have a thickness t, and the vertical walls are 2t.
So that's the expansion process. And the process that's commonly used for honeycombs is called a corrugation process. And for the corrugation process, it's just like the lower schematic here shows.
You take a flat sheet. So you've got a roll of a flat sheet. And you've got some rollers that have the right shape to give you the corrugated profile that you want. You pass the sheet through the rolls and you get individual sheets out.
And each sheet is kind of a half hexagon. And then you put the sheets together and that forms the whole hexagon. So you have a flat sheet that's fed through a shaped wheel to form half hexagonal sheets, which you then bond together.
And it's the same kind of thing that the inclined walls have thickness t and the vertical walls have thickness 2t. And this corrugation process, you can only really use it in materials that you can deform a fairly large amount to get the corrugations. So typically, this is for metals. And aluminum is probably the most common metal that this is used for.
AUDIENCE: How are the corrugated sheets attached to each other?
LORNA GIBSON: I think they're just bonded with epoxy. Yeah, so obviously if you wanted to use it for high temperature performance-- you know, all of these things are bonded with some sort of epoxy or some sort of resin. So there's an issue if you wanted to use it higher temperatures.
So another process that's used to make ceramic honeycombs is an extrusion process. And you just take a ceramic slurry and you pass it through a die. And you can make a ceramic honeycomb by doing that. And I believe that's how they make the ceramic honeycombs I passed around, the catalytic converter ones.
Other techniques involve rapid prototyping. You can 3-D print honeycombs. And Jennifer Lewis has a project on 3-D printing of honeycombs up at Harvard. And one of the interesting things they're looking at is not just printing with an ink, but printing with a fiber reinforced ink. So they're making cell walls of the honeycomb that are fiber reinforced. And one of the tricks is trying to orient the fibers in the way that you want them to be oriented.
So there's rapid prototyping techniques as well. You can use also selective laser centering. Let's call it selective laser scanning. So you can have a photosensitive polymer and use a laser to cure that and build up a honeycomb type structure.
And you can also cast honeycomb structures. So those rubber honeycombs that I pass around, those are made by casting. You take a liquid silicone rubber and you add a hardener and you pour it into a mold.
Another kind of interesting way that people have made-- well here's another example of the honeycombs that are 3-D printed. And this is an example of-- or a couple of examples of looking at a bio carbon template.
So what that means is that these materials are based on the wood, but none of them are actually wood. So what they do is they take wood, like they take pine, or they take beech or something. They take some kind of wood and they carbonize it. So that they do the same processes as used for making carbon fibers.
So you put the wood in an inert atmosphere. And you pyrolyze it. You heat it up to I think 800 degrees C. And all you're left with is the carbon. And it preserves the structure. And you replicate the structure. You just get the same structure. There's some shrinkage. The shrinkage is about 30%. But you get the same structure as the wood.
So this material up here is actually all carbon. It's just replicating the wood that was used, the pine that was used. An then what people have been doing is using that carbon structure and then infiltrating that with gaseous silicon. And they form silicon carbide. So these structures down here are all silicon carbide replicas of wood.
And they're thinking about using that for things like filters for high temperatures or for catalyst carriers. And one of the attractive features is wood has fairly small cells. The cells around 50 microns across. And so you get a large surface area.
And this is a similar thing here. These two are the carbon template. And here they've used silicon and they've actually filled in the voids. And so they've got silicon carbide where the cell walls used to be. And they've got silicon where the void used to be. So people are playing around with this is another way of making a honeycomb type of structure. And they use other kinds of plants besides wood as well. But that's the kind of general idea.
So the idea is that wood has a honeycomb like structure. And the cells are fairly small. the cells are in the order of 50 microns sort of in diameter and maybe a few millimeters long. And this bio carbon template replicates the wood structure.
So the wood is pyrolized at 800 degrees C in an inert atmosphere. So say an inert gas. And that gives you the bio carbon template. And you maintain the structure, although there's some shrinkage. structures
And then this carbon structure can then be further processed. So for example, you can infiltrate it with a gaseous silicon. And you end up with a silicon carbide wood replica.
So possible applications are things like high temperature filters, or catalyst carriers. I think that's it on the honeycombs. Are we good with all sorts of methods?
And my little talk here on processing is certainly not comprehensive. I'm sure there's other ways people have developed. These are some main ways.
All right then, I want to talk about foams as well. People have developed different types of processes for different types of solids, so polymers, and metals, and ceramics. So I just go through each class of solid and talk about that.
So the idea with polymer foams is that you want to introduce gas bubbles into either a liquid monomer or a hot polymer. And then you want the bubbles to grow. And then you want to stabilize them and solidify it by other cross linking or by cooling the hot polymer. So there's a variety of ways of doing that, but let me just put that down.
So there's a few ways to get the bubbles in there in the first place. One, is just by mechanical stirring. So if you've ever made meringue, you know what that is, you just take a whisk and you beat egg whites and bubbles. The air will get enveloped in the egg whites. They also do that with polymers.
Or you can use a blowing agent. And there's several varieties of blowing agents. So the blowing agents are divided into physical and chemical blowing agents. And the physical ones, they force the gas into solution under high pressure, and then you reduce the pressure, and the gas bundles expand.
So you can use physical blowing agents. Or you can introduce liquids that, if you're using a hot polymer, that at the temperature of the hot polymer, they form a gas. So that the liquid just turns into a gas. And that would form vapor bubbles.
And then the chemical blowing agents. There's a couple of different ways that those work. You can either use chemical blowing agents where you have two parts that react together to form a gas. And so that gas then blows the foam. Or you can have a chemical blowing agent that reacts with the polymer to form a gas and that blows the foam. So either way. And you can have them decompose on heating. So the same kind of thing. Evolved the gas when it gets into the hot polymer.
So there's these different ways of blowing the foams. And there's many, many different types of these blowing agents. But, these are kind of the general techniques. And whether or not the foam forms an open cell or a closed cell structure depends on the rheology of the polymers, so the viscosity of it, and also the surface tension.
Another way to make a foam is to make something called a syntactic foam. A syntactic foam is made by taking thin walled hollow spheres and then using, say a resin, like an epoxy resin, to bond them together.
So you end up with something that's porous. And you've got the void from the hollow sphere, but you don't foam it in the same way that you blow bubbles through it in some way.
One other thing about polymer foams is they sometimes have a skin on the surface. So when you blow them, say you've got a mold, there will be a skin that forms against the mold, and sometimes the process is designed in a way that the skin is thick enough that it acts like a skin in a sandwich panel. So they control the mold in a way and the blowing process so that they get a foam in the middle and thicker skins on the top and the bottom surface. And that forms a sandwich panel. Those are called structural foams.
Let's see. So I think what I'm going to do next is the next section's on metal foams. And I've got a few slides on that. So I think I'm going to run through the schematics and just talk about it. But, I'll put the notes on the board next time.
And there is one thing I forgot to do at the beginning. I like to tell you a little about me. And I want to hear about you. So I wanted to leave a few minutes for that. So let me just wait until people are finished writing stuff down. And I'll go through these in a few minutes, and then we'll through it in more detail next time. And I'll write notes down. OK? Are we good?
So there's a whole variety of ways of making foamed metals. And most of them have been focused on aluminum. But you could in theory do them with other types of metals. So this was one of the first processes and it just involved taking a molten aluminum, so here's the aluminum down here in a crucible.
They added silicon carbide powder to it and then they just used a stirring paddle, like they just stirred it up and mixed gas in that way. And they found that they got bubbles that rose up. And then they used conveyor belts to just kind of pull the foam off.
And the thing about the silicon carbide was that if you didn't have that, then the bubbles wouldn't be stable enough that you could do this. The bubbles would collapse before you got to be able to pull them up. But the silicon carbide I think makes the aluminum melt more viscous and it helps prevent sort of drainage and collapse of the bubbles. And so that's one way.
And there's a type of foam called Cymat, and this is an example of the foam that's made with that process. Maybe I'll bring it next time and we can pass it around.
Another method is to use a metal powder and titanium hydride powder. Then you can consolidate that. So here's-- it's hard to see the writing, but this is a aluminum powder. This would be the titanium hydride powder. You mix them together and then you compact them. You press them together.
And the titanium hydride decomposes and forms the hydrogen gas at a temperature at which the aluminum is not really quite molten but it's kind of viscous-y, kind of softening. And so when the aluminum is soft like that and the titanium hydride decomposes and forms the hydrogen gas, you get a foam from that process. And I think, somewhere, yeah, this foam here I think was made by that process.
Then in a similar thing, you can just put titanium hydride powder into molten aluminum, and again the titanium hydride powder evolves the hydrogen gas and you get foamed aluminum from that. And I think this foam here was made with that process. They all look kind of similar.
Another method is by replicating an open cell polymer foam. So I think I passed an open cell polymer foam around. And that's made by taking an open celled-- an open cell aluminum foam-- it's made by taking an open cell polymer foam, you fill up all the voids with sand. You then burn off the polymer, but now you've got sand in all the sort of places where there were voids. And then you infiltrate molten metal into that. And then you get rid of the sand. And then you're left with an aluminum foam that replicates the polymer that you started off with. So this replication technique.
There's a vapor deposition technique. And this was developed by Inco to make nickel foams. So they take again an open cell polymer foam, that's kind of this thing here is, and they infiltrate into it nickel CO4. The only teeny detail that's a problem with this process is that happens to be highly toxic. So they put this gas through here. And then they get nickel depositing on the polymer and they burn the polymer out and they center it.
So it is possible to do this. They have done this. But it's not that practical because of the toxicity of the gas.
Now another method is something called entrapped gas expansion. And here, what you do is you take a can, like a metal can. This one's titanium, a titanium alloy. And then you put a titanium powder in here. You evacuate the can. So the can has a little valve on it, so you can evacuate it. And then you backfill it with argon gas and you pressurize the argon gas.
So you've got a powder with sort of pressurized gas inside of a can. And then you hot isostatically press it. So you heat it up and you press it uniformly in all three directions. And you compact it.
And then, if you want you can roll it. Sometimes people roll it because they want to make sandwich panels, and they want to have a certain thickness, and they want to have faces on the panel. But then you heat that up. And as you heat it up, the gas evolves again. And the thing expands and you get a foam that way. So that's another method.
Another method is by making hollow spheres and then bonding the spheres together. And in this process-- this was developed at Georgia Tech. They used the titanium hydride again. They made a slurry of the titanium hydride in an organic binder in a solvent. And then they had a little kind of needle that they injected gas.
And so they had this slurry and they were blowing gas through this needle and they got hollow spheres of the titanium hydride. And then they heated it up, and again evolved the hydrogen gas off. But now they're just left with titanium spheres.
And then they bonded the spheres together. And these aren't titanium. This is an iron chromium, like it's not quite a stainless steel. But this is the same thing. I can pass that around next time too. Those are the little beads that they make.
And then there's also fugitive phase technique. So you can take say salt particles, put them in a mold and pour a liquid metal into that, and then, leach the salt away. And I think that's it for the metal foams.
So I think I'm going to stop there for today in terms of the lecture. I'll go over that again next time. And I'll write stuff on the board.
But I wanted to tell you a bit about me. So the people in 3032, they already know me because they had me in the fall. But I see some unfamiliar faces. So I thought I would tell you a little about me.
So I grew up in Niagara Falls in Canada, big power station. Lots of big civil engineering works in Niagara Falls.
And my father worked at an engineering company that specialized in the design of hydroelectric power stations. It was founded by the guy who designed the Niagara Falls power station.
And then I went to university in Toronto. And I did a degree in civil engineering in Toronto. And then when I finished my degree in civil engineering, I wasn't really sure what I wanted to do next. And I applied for some jobs, and I applied to graduate school. I applied to MIT. And I didn't get in-- ouch.
But I did OK, it turns out. And I ended up-- I had an advisor when I was in Toronto who had taken a sabbatical in Cambridge, England. And he said he thought I might enjoy Cambridge, England.
And I ended up going to Cambridge, England to doing my PhD there. And I worked on the cellular solids for my PhD.
And it was a nice combination because I was interested in material behavior and mechanics, but I had a background in me in civil engineering. And these are just like civil engineering structures, but they're on a little teeny weeny scale, not like big buildings or bridges or something, like little teeny things.
And really all of this has come out of doing that PhD in Cambridge. But when I was there, I never even thought about being an academic. And I never applied for any academic jobs. I didn't think I wanted to be an academic. And I went and got a job in Calgary in the oil business. And I was working at a consulting firm that did work for the oil business.
I hated it. I just hated it. It was like I had a boss. I hated having this boss. And, you know, the projects were too short term. The winter in Calgary-- if you think this is bad, you've seen nothing. Like less snow, but cold. I mean like 30, 40 below, everyday, cold. Real cold.
So I stayed there one winter. And somewhere along the way there, I decided maybe the academic job thing would be good. And I just sent my CV out to a bunch of Canadian universities. And I ended up getting a job at the University of British Columbia in Vancouver. And I lived in Vancouver for two years.
And I probably would have stayed there, except there was a gigantic recession, and it was all very depressing, and there was no money. And that the universities in Canada are almost all run by the provincial governments. And the government had no money. It was all, you know, frustrating.
And I sort of thought, oh, I'll look around and see what else I can get. And I answered a little ad in Civil Engineering Magazine for a job at MIT. And I got the job at MIT. And I was in the civil engineering department for about 12 years. And then I moved over to the materials department.
Because my work started off on sort of sandwich panels and structural things. And then it kind of became more biomedical stuff and had less and less to do with civil. And I've been in the materials department since then. And this is kind of what I do, this kind of work. So that's kind of my little five minute story.
Free Downloads
Video
- iTunes U (MP4 - 147MB)
- Internet Archive (MP4 - 147MB)
Subtitle
- English - US (SRT)